Products Designed for Targeted Mechanism of Action
After over a decade of research, screening, and discovery work, our founding scientists identified that the ideal natural substances that could be chemically manipulated to meet our rational drug design objectives were the non-psychoactive cannabinoids, cannabidiol (CBD) and cannabigerol (CBG).
CBD has been studied extensively and been shown to have a very good safety profile and health benefits, including anti-inflammatory and anti-oxidative activities. Additionally, CBG has shown to have health benefits and a good safety profile and has demonstrated its ability to act as a neuroprotectant to protect nerve cells in the brain from damage. It improves motor deficits and preserves striatal neurons against 3-nitropropionic acid toxicity.
Our lead product candidates, DHP-101 and DHP-102, are first-in-class potentially disease-modifying therapies, which have been created with same chemical architecture as the natural molecules to achieve the goal of maintaining the basic properties and safety of the natural molecules while adding enhanced and additional effects on other biological receptors and pathways.
DHP–101: A treatment for Multiple Sclerosis & Systemic Sclerosis
The novel active pharmaceutical ingredient in DHP-101 was rationally designed to achieve disease-modifying objectives by being a modulator of the peroxisome proliferator-activated receptor gamma (PPARγ), an agonist of cannabinoid type 2 (CB2) receptor, and an activator of the hypoxia inducible factor (HIF) pathway, as well as other pathways involved in the pathophysiology of autoimmune and neurodegenerative diseases.
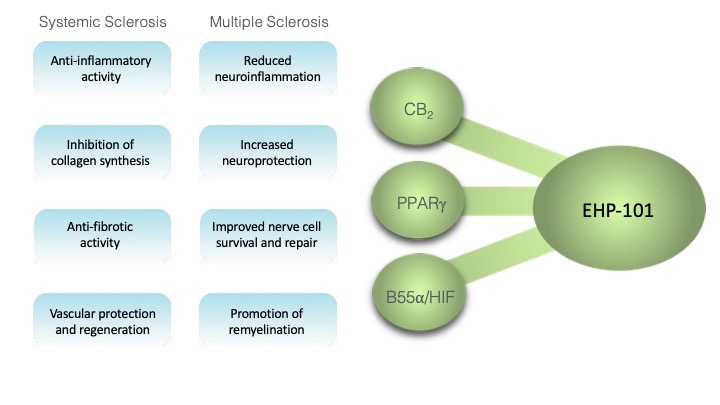
In preclinical studies, DHP-101 demonstrated potential disease-modifying benefits relating to prevention of demyelination and stimulation of remyelination in multiple sclerosis (to see a video of one of these preclinical study results, click here). In systemic sclerosis models, similar disease-modifying benefits were seen, both in reducing inflammation and fibrosis, as well as promoting the protection and regeneration of the vasculature, which is important in systemic sclerosis.
Additionally, in multiple preclinical studies DHP-101 exhibited a good safety profile, including in long-term (6- and 9-month) toxicology studies in rats and dogs. In humans, our Phase I randomized, double-blind, placebo-controlled clinical study of DHP-101 in 104 healthy volunteers also demonstrated good safety and tolerability profiles with no significant adverse effects. No maximum tolerated dose was reached, even at doses many times higher than the anticipated therapeutic dose, providing strong evidence for a flexibility in setting the dosing of patients in future clinical studies.
DHP–102: A treatment for Parkinson’s and Huntington’s disease
DHP-102 is being developed as an oral pharmaceutical product candidate for the treatment of various neurodegenerative diseases. The novel API in DHP-102 was designed to positively affect PPARγ, a key molecular target for the treatment of Huntington’s disease (HD) and Parkinson’s disease (PD), as well as other pathways involved in the pathophysiology of neurodegenerative diseases. It has been shown to activate a transcription factor involved in nerve cell regeneration (neurogenesis), Ctip2. It also reduces the expression of cyclooxygenase-2 (COX-2) in nerve cells (involved in inflammation and pain) and is an activator of the ERK pathway.
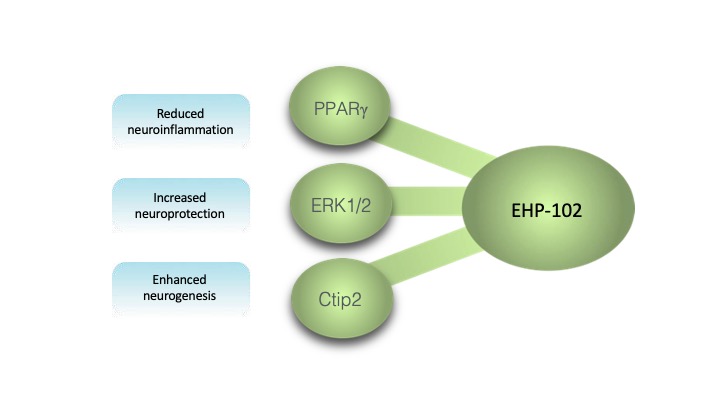
In preclinical studies, DHP-102 demonstrated the regeneration of nerve cells, protection against neuroinflammation and neurodegeneration in Huntington’s disease models, and reduction in the loss of dopamine production in Parkinson’s disease models, which is the main pathophysiologic process in PD. These data support DHP-102’s potential to be disease-modifying in addition to providing functional benefits.

